
What Are Acids And Bases?
- types of compounds that have characteristic formulas and similar chemical behaviours
- very important reactants and catalysts in industrial processes
- acids and bases have opposite properties and have the ability to cancel or neutralize each other
- acids and bases are carefully regulated in the body by the lungs, blood, and kidneys through equilibrium processes
Daily Examples
-Acids:
-
vinegar
-
lemon juice
-
battery acid
-
Coca-cola®
-Bases
-
baking soda
-
ammonia
-
toothpaste
-
Windex®
Characteristics of Acids and Bases
| Acids: | Bases: |
|
|
Theories
- According to the Arrhenius theory, all acids release hydrogen ions (H+) when dissolved in water. For example: HCl + H2O à H+(aq) + Cl-(aq)
- According to the theory, all bases release hydroxide ions (OH-‐) when dissolved in water. For example: NaOH + H2O à Na+(aq) + OH-(aq)
The Bronsted-Lowry Theory
-
An acid is a proton (hydrogen ion) donor.
-
A base is a proton (hydrogen ion) acceptor.
Strong Acids & Bases vs. Weak Acids & Bases
Acids and bases are often referred to as strong or weak.
Acids:
-A strong acid dissociates completely in water. This means that none of the acid is left in solution; rather, 100% of the acid ionizes into a hydrogen ion (H+) and an anion.
-A weak acid does not dissociate completely in aqueous solution.
There are six common strong acids:
-
HCl-hydrochloric acid
-
HNO3-nitric acid
-
H2SO4-sulfuric acid
-
HBr-hydrobromic acid
-
HI-hydroiodic acid
-
HClO4-perchloric acid
Bases:
-A strong base dissociates completely in water to form hydroxide ions (OH-) and a cation.
-A weak base does not dissociate completely in aqueous solution.
There are six common strong bases:
-
LiOH -lithium hydroxide
-
NaOH -sodium hydroxide
-
KOH -potassium hydroxide
-
Ca(OH)2 -calcium hydroxide
-
Sr(OH)2 -strontium hydroxide
-
Ba(OH)2-barium hydroxide
Water
Water, H2O, seems to pose a strange dilemma. When water molecules are ionized, they form equal quantities of hydrogen ions and hydroxide ions, though in reality, hydrogen ions usually attach themselves to another water molecule, forming the hydronium ion.
2H2O à H3O+ + OH-
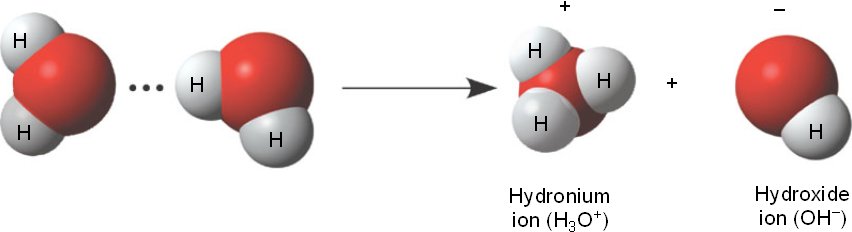
Because of the equal nature of both ions, water is considered to be neutral – it is not acidic or basic. Because both ions are always present in aqueous solution, the only way to determine whether a solution is acidic or basic is to look at the ratio of hydrogen ions and hydroxide ions.

- H+ > OH- à acidic
-
H+ < OH- à basic
-
H+ = OH- à neutral
The pH level of water is 7, meaning it is neutral.
The pH Scale
What is it?
- The pH scale is a logarithmic scale with values ranging from 0-‐14. “Logarithmic” means that a change of one unit on the scale represents a tenfold effect on the pH.
à acid with a pH of 3 is ten times stronger than an acid with a pH of 4
- pH stands for “potenz Hydrogen” (potential for hydrogen), meaning the concentration of hydrogen ions in a solution. An increase of 1 on the scale represents dividing the concentration of H+ ions by 10 (or 101), and a decrease of 2 represents multiplying the concentration by 100 (or 102). A strong acid has a low pH, whereas a strong base has a high pH.
pH values:
- pH < 7 àAcidic
- pH = 7 àNeutral
- pH > 7 àBasic

How to Find the pH
- There are various methods to measure pH, such as using pH paper or a pH meter. If you know the concentration of H+ in solution, you can use the following equation to calculate pH:
à pH = ‐log10 [H+]
(where [H+] is the number of hydrogen ions present in 1 liter of solute, otherwise known as concentration)
- Example:
What is the pH of a solution in which the hydrogen ion concentration is 0.001?
pH = -log10 [H+]
pH = -log10 [0.001]
pH = 3 , the solution is acidic at a pH level of 3
Neutralization Reactions
What type of reaction is the following?
HCl(aq) + NaOH(aq) à H2O(l) + NaCl(aq)
- This is a special type of double displacement reaction that occurs between acids and bases, and is also called a neutralization reaction. These reactions form a salt and often water.
*When a strong base and a strong acid react, they form a neutral solution.
Uses:
- There are many practical uses for neutralization reactions. For example, baking often requires sodium bicarbonate (NaHCO3), or baking soda, and some sort of acid, such as vinegar, cream of tartar, or lemon juice. The baking soda and the acid react to form a gas, which is trapped in the batter and causes the baked good to rise.
- Antacids also take advantage of neutralization reactions. Sometimes the acid in the stomach becomes too concentrated and causes pain. An antacid is a mild base that helps to neutralize the acid and calm the stomach pain.
Creating Acids & Bases
-
When a non-metal is burned in oxygen, it forms a non-metal oxide. A non‐metal oxide reacts in water to form an acid.
C(s) + O2(g) à CO2(g)
CO2(g) + H2O(l) à H2CO3(aq)
- When a metal is burned in oxygen, it forms a metal oxide. A metal oxide reacts in water to form a base.
4K(s) +O2(g) à 2K2O(s)
K2O(s) +H2O(l) à 2KOH(aq)
Acid Precipitation
What is it?
- Acid precipitation is the term used to describe any precipitation (rain, snow, dew, fog), which is more acidic than normal.
- All precipitation is normally slightly acidic, with normal rain having a pH of about 5.6. The slight acidity is due to the carbon dioxide naturally present in the atmosphere. The gas reacts with water in the following way:
CO2(g) + H2O(l) à H2CO3(aq)
- However, the increased acidity is due mostly to sulfur dioxide (SO2) and nitrogen oxides (NOx). These gases are transformed into sulfuric acid (H2SO4), nitric acid (HNO3) and nitrous acid (HNO2) when they come into contact with water in the atmosphere.


-As shown on the pH scale, acid rain is quite acidic.
Where do these Pollutants Come From?
- Sulfur dioxide is a by‐product of industrial processes and burning of fossil fuels. Ore smelting, coal‐fired power generators and natural gas processing are the main contributors. In eastern Canada, over 50% of the acid deposition originates from the United States.
- Nitrogen oxide emissions come from combustion of fuels in motor vehicles, residential and commercial furnaces, industrial and electrical-utility boilers and engines, and other equipment. Again, U.S. emissions have a huge impact on Canadian atmosphere.
Effects
Humans
-
sulfur dioxide reacts with water vapour to form fine particles of sulfate these particles contribute to smog, and can also become lodged in the respiratory tract and contribute to asthma and other respiratory problems
-
acid precipitation can damage buildings and statues leading to expensive repairs
-
acid precipitation ruins car finishes by ‘eating away’ at the paint
Forests
-
acid precipitation damages the surfaces of leaves and needles, reducing a tree's ability to withstand cold, and inhibiting plant germination and reproduction
- acid precipitation causes leaching of soil nutrients
Aquatic Environments
-
slight increases in acidity can kill crustaceans, insects and some plankton species, disrupting the food web of lakes
-
higher increases in acidity can kill fish species or prevent them from reproducing

Check these out!
http://www.youtube.com/watch?v=MqHw1hMEkAQ
http://www.youtube.com/watch?v=oJAbATJCugs
Preventing Acid Precipitation
There are many strategies for reducing sulfur dioxide and nitrogen oxide emissions. The Canadian government has been working on this since the launch of The Eastern Canada Acid Rain program in 1985. Sulfur dioxide emissions have decreased significantly since this time, and acid precipitation has also decreased. The cooperation of the U.S. was also needed, and in 1991 the Canada-US Air Quality Agreement was signed.
Industrial strategies to reduce emissions consist of coal cleaning, burning low-‐sulfur coal, using catalytic converters to convert oxides to harmless gases, and several other techniques.
Individuals can do their part by using less electricity, using public transportation, and maintaining their vehicles.
Angela De Jong, manageBAC, 2012, http://www.turnerfenton.managebac.com (accessed May 18, 2012)